Anavar (oxandrolone) oral steroid is an androgen and synthetic anabolic steroid (AAS) medication. It helps promote weight gain in various situations. It helps offset protein catabolism caused by long-term corticosteroid therapy. It supports recovery from severe burns. It treats bone pain linked to osteoporosis. It aids in the development of girls with Turner syndrome. It is used for other indications. It is taken by mouth. It was sold under the brand name Oxandrin, among others. You can purchase this steroid from the UK Steroid Shop.
To understand Anavar—Oxandrolone more fully, it’s essential to grasp the fundamentals of Anabolic Androgenic Steroids (AAS). These steroids are derivatives of the hormone testosterone, developed to enhance the body’s ability to build and synthesise molecules—an essential process known as anabolism. AAS were crafted to maximise the anabolic effects, such as promoting protein synthesis, preventing protein breakdown, retaining nitrogen, and building skeletal muscle, while minimising the androgenic effects typically linked to puberty changes.
The drug is a synthetic androgen and anabolic steroid. Hence, it is an agonist of the androgen receptor (AR). This receptor is the biological target of androgens such as testosterone and dihydrotestosterone. Side effects of oxandrolone include severe cases of peliosis hepatis, sometimes linked with liver failure and intra-abdominal haemorrhage. It can also cause liver tumours, which can sometimes be fatal. Additionally, it causes blood lipid changes associated with increased risk of atherosclerosis. Additional warnings include the risks associated with cholestatic hepatitis. Hypercalcemia can occur in patients with breast cancer. There is an increased risk for the development of prostatic hypertrophy and prostatic carcinoma in older patients.
AAS like Anavar (oxandrolone) are designed with a high anabolic-to-androgenic ratio, making them particularly effective for muscle building while presenting fewer androgenic side effects. For example, oxandrolone is known for its strong anabolic effects and weak androgenic effects, which result in a mild side effect profile. This combination makes it especially suitable for use in women, where milder side effects might include an increased sexual desire and symptoms of hyperandrogenism, such as acne. Women may also experience symptoms of masculinisation, such as increased hair growth and voice changes.
Understanding the balance between anabolic and androgenic effects is crucial for anyone considering AAS. Oxandrolone, with its specific profile, offers a tailored approach to those needing therapeutic benefits with minimised side effects.
Anavar—oxandrolone—was first described in 1962 and introduced for medical use in 1964. The drug was a controlled substance in many countries. Non-medical use for purposes such as improving physique and performance has generally been illicit. In the United States, the FDA withdrew the approval for the drug in 2023. This decision was for reasons of safety or effectiveness. The withdrawal followed a 2019 letter from Gemini, a drug manufacturer, stating that the product was no longer being marketed.
Like other AASs, oxandrolone may worsen hypercalcemia by increasing osteolytic bone resorption. When taken by pregnant women, oxandrolone may have unintended effects, such as masculinisation, on the foetus.
Medications Contraindicated with Oxandrolone (Anavar) Use
While considering oxandrolone, it’s crucial to be aware of certain medications that may interact adversely. Here’s a detailed look:
- Anticoagulants: Anabolic steroids, such as oxandrolone, can enhance the activity and sensitivity of oral anticoagulants (blood thinners), potentially leading to increased bleeding risks.
- Oral Hypoglycemic Medications: These medications, used to manage blood sugar levels, may interact with oxandrolone, requiring careful monitoring and possible dosage adjustments.
- Adrenal Steroids or ACTH: Combining these with oxandrolone can amplify hormonal effects, necessitating medical supervision.
Understanding these interactions is vital for safe oxandrolone use, especially for individuals with pre-existing health conditions or those on multiple medications. Always consult with a healthcare professional to tailor the treatment to your specific needs.
There were severe cases of peliosis hepatis, sometimes associated with liver failure and intra-abdominal haemorrhage. There were also instances of liver tumours, sometimes fatal. Changes in blood lipid profiles associated with increased risk of atherosclerosis led the FDA to remove approval in June 2023. Additional warnings include the risks associated with cholestatic hepatitis. There is also the risk of hypercalcemia in patients with breast cancer. Additionally, there is increased risk for the development of prostatic hypertrophy and prostatic carcinoma in older patients.
Hepatic Effects of Oxandrolone
All anabolic androgenic steroids, including oxandrolone, impart a hepatic effect. Prolonged use of Anavar, particularly for periods greater than one year, has been shown to result in hepatic dysfunction. This dysfunction can manifest as:
- Elevations in liver function enzymes: A marker of liver stress or damage.
- Peliosis hepatis: A rare condition where blood-filled cysts form in the liver.
- Adenomas: Benign liver tumours that can potentially become cancerous.
- Hepatocellular carcinoma: Although not conclusively proven, concerns exist about the potential development of liver cancer.
While short-term use (less than 3 months) has not been conclusively linked to severe hepatotoxicity, caution is advised due to the potential for serious liver conditions with prolonged use. Understanding these risks is crucial for making informed decisions regarding the use of oxandrolone.
Medical uses
Medical Uses
Anavar (oxandrolone) has been researched and prescribed as a treatment for a wide variety of conditions. It was FDA-approved for treating bone pain associated with osteoporosis. It aids weight gain following surgery or physical trauma. It is also used during chronic infection or in the context of unexplained weight loss. Additionally, it counteracts the catabolic effect of long-term corticosteroid therapy. Anavar—the oral steroid oxandrolone—is used to quicken recovery from severe burns.
Anabolic androgenic steroids are indicated in patients with chronic wasting conditions, such as the loss of muscle mass. These conditions include sarcopenia, AIDS-related muscle wasting, severe burn injury, trauma following surgery, and other catabolic disorders. Oxandrolone is approved by the FDA for the treatment of patients with prolonged use of corticosteroids to prevent protein catabolism. It has also been used to promote weight gain after extensive surgery, during chronic infectious states, or after severe trauma.
Numerous clinical trials have demonstrated the therapeutic advantages of oxandrolone in the management of severe burn injuries. It was widely adopted as a standard treatment protocol in burn centres globally. Meta-analyses of clinical trials substantiate the efficacy of oxandrolone in severe burn cases. The benefits are manifold and significant. They include a reduction in catabolic weight loss. They also include augmentation of lean body mass. Furthermore, there is an enhancement of donor-site wound healing. Lastly, there is a decrease in the duration of both intensive care unit (ICU) and overall hospital stay. These benefits do not appear to be accompanied by an increased risk of infection. They do not seem to cause hyperglycemia or hepatic dysfunction either. This underscores the safety profile of oxandrolone in the severe burn patient population. Data analysis confirms oxandrolone’s significant advantage in promoting skin healing as an adjunct therapy for adult burn patients.
Anavar (oxandrolone) improves weight regain, bone mineral density, and lean body mass and accelerates wound healing for donor graft sites. It is recommended as an adjunctive therapy alongside insulin, metformin, and closely monitored propranolol. It is intended for severe burn patients for metabolic and nutritional support. Oxandrolone improves both short-term and long-term outcomes in people recovering from severe burns. It is well-established as a safe treatment for this indication. One of the underlying mechanisms in burn management is that oxandrolone helps reduce hypermetabolic response. This response is characterised by increased energy expenditure. It includes elevated stress hormone levels such as cortisol. Additionally, it includes insulin resistance, muscle wasting, and impaired wound healing. This response is reduced by improving whole-body nitrogen balance. It also helps in preserving lean body mass during recovery.
As of 2019, oxandrolone was prescribed off-label for the development of girls with Turner syndrome. It was also used to counteract wasting of diverse origin.
As of 2012, oxandrolone was used in the treatment of idiopathic short stature, anaemia, hereditary angioedema, hypogonadism and alcoholic hepatitis.
Medical research established the effectiveness of oxandrolone in aiding the development of girls with Turner syndrome. Oxandrolone had long been used to accelerate growth in children with idiopathic short stature. It is unlikely to increase adult height. In some cases, it may even decrease it. As of 2015, oxandrolone has largely been replaced by growth hormone for this use. However, a 2019 Cochrane review compared the effects of adding oxandrolone to growth hormone treatment to growth hormone alone. It found moderate-quality evidence. The addition of oxandrolone led to an increase in the final adult height of girls with Turner syndrome. Low-quality evidence showed no increase in adverse effects. When the same review assessed the effects of adding oxandrolone to growth hormone treatment on speech, cognition, and psychological status, the results were inconclusive. The review found the results to be inconclusive. The evidence was of very low quality. Children with idiopathic short stature or Turner syndrome were given doses of oxandrolone far smaller than those given to people with burns to minimise the likelihood of virilisation and premature maturation.
Oxandrolone shows positive effects on cardiometabolic health and visual, motor, and psychosocial functions in adolescent males with preserved testosterone production. This is seen in those with Klinefelter syndrome.
Oxandrolone can also reduce males’ fertility, another side effect common among androgens. In an attempt to compensate for the exogenous increase in androgens, the body may reduce testosterone production via testicular atrophy and inhibition of gonadotropic activity.
The use of Anavar—oxandrolone—significantly impacts testosterone production by disrupting the natural pituitary axis. This disruption results in a decrease in key hormones such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are crucial for testosterone synthesis.
Additionally, it reduces the levels of Sex Hormone Binding Globulin (SHBG). This reduction is noteworthy because it causes an increase in free testosterone—the active form of testosterone in the body—while paradoxically decreasing total testosterone levels.
These hormonal shifts can have profound effects, especially in men with chronic conditions like muscle wasting or HIV wasting, where Anavar is often combined with supplementary testosterone to counterbalance its suppressive effects on natural testosterone production.
Understanding these dynamics is essential for managing the potential side effects associated with oxandrolone, ensuring that users can make informed decisions about its use.
Oxandrolone can also reduce male fertility, another side effect common among androgens. In an attempt to compensate for the exogenous increase in androgens, the body may reduce testosterone production via testicular atrophy and inhibition of gonadotropic activity.
Anavar—Oxandrolone usage decreases the natural pituitary axis necessary for maintaining testosterone production. Users will experience a decrease in key hormones such as LH (luteinizing hormone), FSH (follicle-stimulating hormone), and SHBG (sex hormone-binding globulin), as well as both total and free testosterone levels.
Effects on Hormone Levels:
- LH and FSH Reduction: These hormones are crucial for stimulating testosterone production in the testes. A decrease can lead to diminished testosterone synthesis.
- SHBG Decrease: While a reduction in SHBG can increase the amount of free (active) testosterone in the body, it also indicates a disruption in hormonal balance, affecting overall testosterone levels.
In cases of chronic muscle-wasting conditions or HIV wasting, Anavar is often combined with testosterone to mitigate these effects. The reduction in SHBG may increase free testosterone temporarily, but the overall impact often leads to lower natural testosterone production.
Non-medical uses
Oxandrolone has been used illicitly by bodybuilders and athletes for its muscle-building effects as a doping agent in sports. Cases of doping with oxandrolone by professional athletes have been reported. Because it is more anabolic than androgenic, women and those seeking less intense steroid regimens used it particularly often.
Contraindications
Like other AASs, oxandrolone may worsen hypercalcemia by increasing osteolytic bone resorption. When taken by pregnant women, oxandrolone may have unintended effects, such as masculinization on the foetus.
Side effects
As of 2004 it was thought that, “uniquely” among 17α-alkylated AASs, oxandrolone showed little to no hepatotoxicity. This was believed to be true even at high doses. However, elevated liver enzymes have been observed in some people. This is particularly true with high doses and/or prolonged treatment. The enzymes sometimes return to normal ranges following discontinuation. The lack of hepatotoxicity turned out not to be true in the long run. There were severe cases of peliosis hepatis, sometimes associated with liver failure and intra-abdominal hemorrhage. There were also instances of liver tumors, sometimes fatal. Changes in blood lipid profiles associated with increased risk of atherosclerosis led the FDA to remove approval in June 2023. Additional warnings include the risks associated with cholestatic hepatitis. There is also the risk of hypercalcemia in patients with breast cancer. Additionally, there is increased risk for the development of prostatic hypertrophy and prostatic carcinoma in older patients.
Women who are administered oxandrolone may experience virilisation. This includes the irreversible development of masculine features such as voice deepening, hirsutism, menstruation abnormalities, male-pattern hair loss, and clitoral enlargement. Because of these side effects, doses given to women and children are minimized. People are usually monitored for virilization and growth abnormalities. Like other androgens, oxandrolone can cause or worsen acne and priapism (unwanted or prolonged erections). Oxandrolone can also reduce males’ fertility, another side effect common among androgens. In an attempt to compensate for the exogenous increase in androgens, the body may reduce testosterone production via testicular atrophy and inhibition of gonadotropic activity.
Unlike some AASs, oxandrolone does not generally cause gynaecomastia because it is not aromatized into estrogenic metabolites. However, although no reports of gynecomastia were made in spite of widespread use, oxandrolone was reported in a publication in 1991. It was associated with 33 cases of gynecomastia in adolescent boys treated with it for short stature. The gynecomastia developed during oxandrolone therapy in 19 of the boys. It developed after the therapy was completed in 14 of the boys. Ten of the boys had transient gynecomastia. Twenty-three had persistent gynecomastia that necessitated mastectomy. Though transient gynaecomastia is a natural and common occurrence in pubertal boys. The gynaecomastia associated with oxandrolone was of a late/delayed onset. It was persistent in a high percentage of the cases. As such, the researchers stated. Although oxandrolone cannot be implicated as stimulatory in gynaecomastia. A possible relationship should be considered in clinicians using oxandrolone in adolescents for growth stimulation.
Interactions
As of 2004 it was known that Anavar—oxandrolone—greatly increases warfarin’s blood-thinning effect, sometimes dangerously so. In April 2004, Savient Pharmaceuticals published a safety alert through the FDA warning healthcare professionals of this. Oxandrolone also inhibits the metabolism of oral hypoglycemic agents. It may worsen edema when taken alongside corticosteroids or adrenocorticotropic hormone.
Pharmacology
Pharmacodynamics
| Medication | Ratios |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
Like other AASs, Anavar (oxandrolone) is an agonist of the androgen receptor, similar to androgens such as testosterone and DHT. The relative binding affinity of oxandrolone for the androgen receptor is about 0.3% of that of metribolone. Activation of the androgen receptor stimulates protein synthesis, which increases muscle growth, lean body mass, and bone mineral density.
Compared to testosterone and many other AASs, oxandrolone is less androgenic relative to its strength as an anabolic. Oxandrolone has as much as 6 times the anabolic potency of testosterone. Still, it has significantly reduced androgenic potency in comparison: oxandrolone exhibits significantly lower virilising androgenic properties compared to testosterone, with a relative potency of only 5%.
Anabolic and Androgenic Effects
Oxandrolone, commonly known as Anavar UK, was specifically designed to maximise anabolic effects while minimising unwanted androgenic effects. This design makes it a preferred choice for those aiming to enhance muscle size and strength without the typical side effects associated with more androgenic steroids.
Specific Benefits of Oxandrolone:
- Increased Muscle Size and Strength: It is known to significantly boost muscle growth and enhance physical strength.
- Enhanced Nitrogen Retention: This promotes a more favourable environment for muscle preservation and growth.
- Reduced Fat Deposition: Users may experience a leaner physique due to Anavar’s ability to lower fat accumulation.
- Protein Synthesis: enhances protein synthesis, vital for muscle repair and growth.
Quantitative Comparison:
- Anabolic: Androgenic Ratio: boasts a ratio of 10:1, far surpassing testosterone’s 1:1 ratio, underscoring its powerful anabolic effects with minimal androgenic impact.
- Steroid Protein Activity Level (SPAI): SPAI is 2.8, indicating a higher efficiency in promoting anabolic activity compared to testosterone’s SPAI of 1.
By understanding these differences, users can better appreciate why Anavar is often chosen for its anabolic benefits with fewer androgenic side effects compared to testosterone.
Compared to methyltestosterone, oxandrolone has about 322 to 633% of the anabolic potency and 24% of the androgenic potency.
The reduced ratio of anabolic to androgenic activity of oxandrolone often motivates its medical use in children and women. Less androgenic effect implies less risk of virilisation. The bodybuilding community also considers this fact when choosing between AASs.
As of 2003 and 2011, oxandrolone was thought to be “uniquely” far less hepatotoxic than other 17α-alkylated AASs, which was thought to be due to differences in metabolism. This turned out not to be the case in the long run, which is why it was taken off the US market in 2023.
Steroid configuration
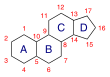
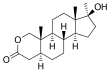
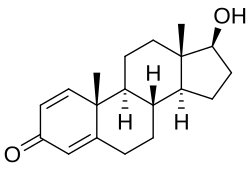
Oxandrolone is based on the tetracyclic steroid framework, which consists of three cyclohexane rings (A, B, and C) and one cyclopentane ring (D). This is a common structure shared by all steroids.
The oxygen atom in the lactone bridge replaces a carbon atom at position 2 of the steroid nucleus, classifying oxandrolone as a 2-oxa-steroid. There is a hydroxyl group (-OH) attached at stereo-direction β to carbon 17, which is a characteristic of 17β-hydroxy-steroids.
The overall structure of oxandrolone is distinguished by these modifications to the standard steroid skeleton. These modifications contribute to its unique properties as an anabolic steroid. The presence of the lactone bridge, i.e., the 2-oxa-steroid classification, is particularly noteworthy, as it is not commonly found in the steroid family. This structural element is what gives oxandrolone its distinctive chemical identity within the class of anabolic steroids. Due to this lactone bridge, oxandrolone is resistant to inactivation by 3α-hydroxysteroid dehydrogenase in skeletal muscle. This is in contrast to DHT and is thought to underlie the preserved anabolic potency with oxandrolone.
As oxandrolone is already 5α-reduced (has a single bond between carbons 4 and 5), it is not a substrate for the 5α-reductase enzyme. Because of this reduction, it is not a substrate for the enzyme. Hence, it is not potentiated in androgenic tissues such as the skin, hair follicles, and prostate gland. This is involved in its reduced ratio of anabolic to androgenic activity. Because it is 5α-reduced, oxandrolone is not a substrate for aromatase; hence, it cannot be aromatised into metabolites with oestrogenic activity. Oxandrolone similarly possesses no progestogenic activity.
Pharmacokinetics
The oral bioavailability of oxandrolone is 97%, indicating that nearly all of the drug is absorbed into the bloodstream when taken orally. This medication is absorbed across the gastrointestinal tract, with peak serum concentrations occurring within 1 hour after ingestion, ensuring rapid efficacy.
Oxandrolone’s plasma protein binding ranges from 94 to 97%, contributing to its stability and resistance to breakdown by the liver. Unlike other anabolic androgenic steroids (AAS), oxandrolone is metabolised primarily by the kidneys and to a lesser extent by the liver. This unique metabolic pathway is thought to contribute to its diminished hepatotoxicity relative to other AASs.
Its elimination half-life is reported as 9.4 to 10.4 hours, extending to 13.3 hours in the elderly. Approximately 28% of an oral dose is eliminated unchanged in the urine, and an additional 3% is excreted in the faeces. This detailed pharmacokinetic profile underscores oxandrolone’s efficient absorption and metabolism, making it a favoured choice among users seeking minimal liver strain.
Chemistry
Oxandrolone is a synthetic androstane steroid and a 17α-alkylated derivative of DHT. It is also known as 2-oxa-17α-methyl-5α-dihydrotestosterone (2-oxa-17α-methyl-DHT) or as 2-oxa-17α-methyl-5α-androstan-17β-ol-3-one and is DHT with a methyl group at the C17α position and the C2 carbon replaced with an oxygen atom. Closely related AASs include the marketed AAS mestanolone (17α-methyl-DHT). There is also oxymetholone (2-hydroxymethylene-17α-methyl-DHT) and stanozolol (a 2,3-pyrazole A-ring-fused derivative of 17α-methyl-DHT). The never-marketed/designer AASs include desoxymethyltestosterone (3-deketo-17α-methyl-δ2-DHT), methasterone (2α,17α-dimethyl-DHT), methyl-1-testosterone (17α-methyl-δ1-DHT), and methylstenbolone (2,17α-dimethyl-δ1-DHT).
History
Oxandrolone was first made by Raphael Pappo and Christopher J. Jung while at Searle Laboratories, now part of Pfizer, and they first described the drug in 1962. They were immediately interested in oxandrolone’s very weak androgenic effects relative to its anabolic effects. It was introduced as a pharmaceutical drug in the United States in 1964.
It was prescribed to promote muscle regrowth in disorders that cause involuntary weight loss and is used as part of treatment for HIV/AIDS. It had also been shown to be partially successful in treating cases of osteoporosis. However, in 1989, in part due to bad publicity from its illicit use by bodybuilders, production of Anavar was discontinued by Searle Laboratories. It was picked up by Bio-Technology General Corporation, which changed its name to Savient Pharmaceuticals. In 1995, following successful clinical trials, Savient released it under the brand name Oxandrin. As of 2011, BTG subsequently had won approvals for orphan drug status by the Food and Drug Administration for treating alcoholic hepatitis, Turner syndrome, and HIV-induced weight loss and as an offset to protein catabolism caused by long-term administration of corticosteroids.
Society and culture
Generic names
Oxandrolone is the generic name of the drug and its INNTooltip International Nonproprietary Name, USANTooltip United States Adopted Name, USPTooltip United States Pharmacopoeia, BAN Tooltip British Approved Name, DCF Tooltip Dénomination Commune Française, DCITTooltip Denominazione Comune Italiana, and JANTooltip Japanese Accepted Name, while ossandrolone is or was formerly the DCITTooltip Denominazione Comune Italiana.
Brand names
The original brand name of oxandrolone was Anavar, which was marketed in the United States and the Netherlands. This product was eventually discontinued and replaced in the United States with a new name, Oxandrin. As of 2011, it was the sole remaining brand name for oxandrolone in the United States. Oxandrolone has also been sold under the brand names Antitriol (Spain), Anatrophill (France), Lipidex (Brazil), and Lonavar (Argentina, Australia, and Italy). Other names include Protivar and Vasorome (Japan). As of 2016, among those using oxandrolone for nonmedical purposes, it has been referred to colloquially as “Var.” This is a shortened form of the old brand name Anavar. Additional brand names existed for products that were manufactured for the steroid black market.
Availability
United States
As of 2017, oxandrolone was one of the few AASs that remained available for medical use in the United States.
In June 2023, the FDA formally withdrew approval for oxandrolone for all indications. The agency stated that possible adverse effects of the drug were sufficiently serious to warrant removal from the US market. The FDA decision was for reasons of safety or effectiveness. This move followed a 2019 letter from Gemini, a drug manufacturer. The letter announced the discontinuation of the product’s marketing.
As of August 2023, the AASs that remained available for medical use in the US were testosterone. Testosterone cypionate, testosterone enanthate, and testosterone undecanoate were also available. Additionally, methyltestosterone, fluoxymesterone, and oxymetholone remained available.
Other countries
Outside of the United States, the availability of oxandrolone is also quite limited. It is no longer available in Europe. Oxandrolone is available in some less-regulated markets in Asia, such as Malaysia, and in Mexico.
Historically, oxandrolone has been marketed in Argentina, Australia, Brazil, France, Italy, Japan, and Spain. But it doesn’t seem to be accessible anymore in these nations.
Legal status
In the United States, oxandrolone was categorised as a Schedule III controlled substance. This was under the Controlled Substances Act along with many other AASs. In 2017, it was a Schedule IV controlled substance in Canada. It was also a Schedule 4 controlled drug in the United Kingdom.
Buy Steroids UK from UK Steroids Shop—Quantum Steroids UK